Class 11 Organic Chemistry 2 Marks important questions
You will get 1 question from these below mentioned 11 questions.
ORGANIC CHEMISTRY for IPE MARCH 2025
IUPAC Names :
|
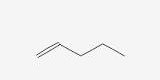
1-PENTENE |
|
|
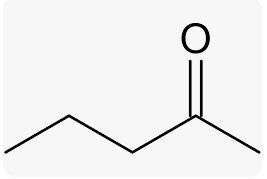
2-PENATNONE |
|
|
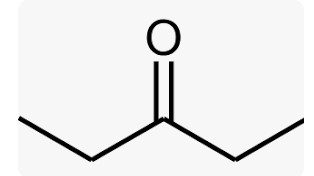
3-PENATNONE |
|
|
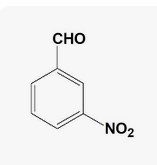
Meta nitro Benzaldehyde |
|
|
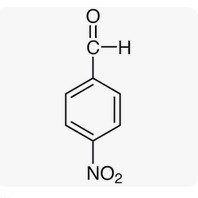
Para nitro Benzaldehyde |
|
|
Trichloro Ethanoic Acid |
|
|
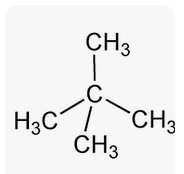
Neo pentane |
|
|
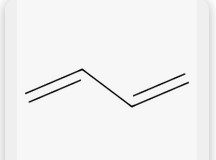
1,3 buta diene |
|
|
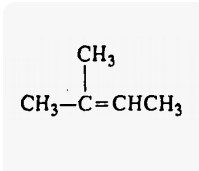
2-Methyl 2-butene |
|
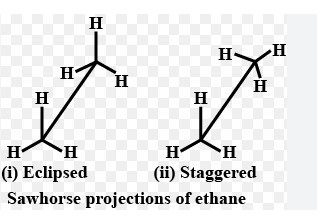
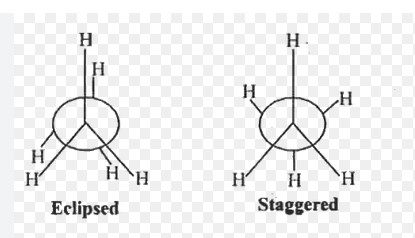
2.Draw the Conformations of Ethane ?
Ethane has two Conformations
1.Eclipsed and
2.Staggered Conformation.
Both can be represented in Sawhorse and Newmann projection formula
Class 11 Organic Chemistry-PDF
Class 11 Organic Chemistry multiple choice questions For EAPCET
😊😊
What is the IUPAC name of CH₃CHO?
- (a) Acetaldehyde
- (b) Methylaldehyde
- (c) Formyl chloride
- (d) Ethanal
Answer: (a) Acetaldehyde
Which of the following behaves both as a nucleophile and as an electrophile?
- (a) CH₃C≡N
- (b) CH₃OH
- (c) CH₂=CHCH₃
- (d) CH₃NH₂
Answer: (a) CH₃C≡N
Which of the following cannot be represented by resonance structures?
- (a) Dimethyl ether
- (b) Nitrate anion
- (c) Carboxylate anion
- (d) Toluene
Answer: (a) Dimethyl ether
What is the hybridization of CH₃CH₂CH₂CN?
- (a) sp, sp³
- (b) sp², sp³
- (c) sp
- (d) sp, sp²
Answer: sp³ for CH₃CH₂CH₂ and sp for CN.
Which one is the strongest acid among the following options?
- (a) CH₂FCOOH
- (b) CH₂ClCOOH
- (c) CHCl₂COOH
- (d) CHF₂COOH
Answer: (d) CHF₂COOH
The displacement of electrons in a multiple bond in the presence of an attacking reagent is called:
- (a) Inductive effect
- (b) Electromeric effect
- (c) Resonance
- (d) Hyperconjugation
Answer: (b) Electromeric effect
During Lassaigne’s test, sulfur and nitrogen present in an organic compound change into:
- (a) Na₂S and NaCN
- (b) Na₂SO₄ and NaCN
- (c) Na₂S and NaCNO
- (d) NaCN and NaCNO
Answer: (a) Na₂S and NaCN
How many σ and π bonds are present in the molecule N≡C-CH-C≡N?
- (a) σ = 5; π = 4
- (b) σ = 6; π = 3
- (c) σ = 4; π = 2
- (d) σ = 3; π = 5
Answer: σ = 5; π = 4
Which one is the correct order of acidity?
- (a) CH₂=CH₂ > CH=CH₂ > CH₃C≡CH > CH≡C
- (b) CH≡CH > CH₃C≡CH > CH₂=CH₂ > CH₃−CH₃
- (c) CH≡CH > CH₂=CH₂ > CH₃C≡CH > CH₃−CH₃
- (d) CH₃−CH₃ > CH₂=CH₂ > CH₃−C≡CH > CH≡CH
Answer: (b) CH≡CH > CH₃C≡CH > CH₂=CH₂ > CH₃−CH₃
σ bonds are stronger than π bonds.
- (a) True
- (b) False
Answer: True
- What is the IUPAC name of CH₃COCH₃?
- (a) Propan-2-one
- (b) Butan-2-one
- (c) Ethanol
- (d) Acetone
Answer: (d) Acetone
- Which of the following is an example of an alkene?
- (a) C₂H₆
- (b) C₃H₆
- (c) C₄H₁₀
- (d) C₅H₁₂
Answer: (b) C₃H₆
- What is the functional group of alcohols?
- (a) –OH
- (b) –COOH
- (c) –CHO
- (d) –NH₂
Answer: (a) –OH
- Which compound is a ketone?
- (a) CH₃CHO
- (b) CH₃COCH₂CH₃
- (c) CH₃CH₂OH
- (d) CH₃C≡CH
Answer: (b) CH₃COCH₂CH₃
- Which of the following has a triple bond?
- (a) Ethylene
- (b) Acetylene
- (c) Propylene
- (d) Butane
Answer: (b) Acetylene
- What is the IUPAC name of C₂H₅Br?
- (a) Bromoethane
- (b) Ethyl bromide
- (c) Bromobutane
- (d) Propyl bromide
Answer: (a) Bromoethane
- Which of the following compounds is aromatic?
- (a) Cyclohexane
- (b) Benzene
- (c) Cyclopentene
- (d) Butadiene
Answer: (b) Benzene
- What type of reaction occurs when an alkene reacts with H₂ in the presence of a catalyst?
- (a) Addition reaction
- (b) Elimination reaction
- (c) Substitution reaction
- (d) Rearrangement reaction
Answer: (a) Addition reaction
- Which of the following statements about alkanes is true?
- (a) They are unsaturated hydrocarbons.
- (b) They contain at least one double bond.
- (c) They are saturated hydrocarbons.
- (d) They have higher reactivity than alkenes.
Answer: (c) They are saturated hydrocarbons.
- What is the main product when ethene reacts with bromine water?
- (a) Ethanol
- (b) 1,2-Dibromoethane
- (c) Ethyl bromide
- (d) Bromobenzene
Answer: (b) 1,2-Dibromoethane
- Which of the following compounds is a primary alcohol?
- (a) CH₃CH₂OH
- (b) CH₃CHOHCH₃
- (c) CH₃C(=O)(OH)
- (d) CH₃C(CH₃)(OH)(C₂H₅)
Answer: (a) CH₃CH₂OH
- What type of isomerism is shown by butanol and isobutanol?
- (a) Structural isomerism
- (b) Geometric isomerism
- (c) Optical isomerism
- (d) Functional group isomerism
Answer: (a) Structural isomerism
- Which reagent can be used to convert an alkene to an alcohol?
- (a) H₂O in presence of acid
- (b) HBr
- (c) NaBH₄
- (d) KMnO₄
Answer: (a) H₂O in presence of acid
- Which compound has a carbonyl group?
- (a) Ether
- (b) Aldehyde
- (c) Alkane
- (d) Alkene
Answer: (b) Aldehyde
- What type of bond exists between carbon atoms in alkenes?
- (a) Single bond only
- (b) Double bond only
- (c) Triple bond only
- (d) Both single and double bonds
Answer: (b): Double bond only
- Which one is a characteristic reaction of aldehydes?
– (a): Reduction to alcohols
– (b): Oxidation to carboxylic acids
– (c): Nucleophilic addition
– (d): All of the above
Answer: (d): All of the above - The process of converting an alkene into an alkane by adding hydrogen is called:
– (a): Hydrogenation
– (b): Halogenation
– (c): Hydrolysis
– (d): Dehydrogenation
Answer: (a): Hydrogenation - Which compound contains a carboxylic acid functional group?
– (a): CH₄
– (b): CH₃COOH
– (c): C₂H₂
– (d): C₂H₅OH
Answer: (b): CH₃COOH - What type of reaction occurs when an alcohol reacts with a carboxylic acid to form an ester?
– (a): Condensation reaction
– (b): Hydrolysis
– (c): Substitution reaction
– (d): Addition reaction
Answer: (a): Condensation reaction - Which of the following compounds has a higher boiling point due to hydrogen bonding?
– (a): Ethane
– (b): Ethanol
– (c): Propene
– (d): Butane
Answer: (b): Ethanol - Which reagent can oxidize primary alcohols to aldehydes?
– (a): KMnO4
– (b): H2SO4
– (c): NaBH4
– (d): LiAlH4
Answer: (a): KMnO4 - The simplest alkyne is:
– (a): Ethyne
– (b): Propyne
– (c): Butyne
– (d): Methyne
Answer: (a): Ethyne - Which compound can undergo hydrolysis to form an alcohol and a carboxylic acid?
-(a): Ester
-(b): Ether
-(c): Alkane
-(d): Alkyne
Answer:(a): Ester - The molecular formula for benzene is:
-(a): C6H6
-(b): C5H5
-(c): C7H8
-(d): C8H10
Answer:(a): C6H6 - Which of the following reactions involves the formation of a cyclic compound from an alkene?
-(a): Polymerization
-(b): Diels-Alder reaction
-(c): Hydrogenation
-(d): Halogenation
Answer:(b): Diels-Alder reaction - In which type of organic compound would you find a phenolic hydroxyl group (-OH)?
-(a): Alcohols
-(b): Phenols
-(c): Ethers
-(d): Aldehydes
Answer:(b): Phenols - What type of hybridization occurs in carbon atoms involved in a triple bond?
-(a): sp² hybridization
-(b): sp³ hybridization
-(c): sp hybridization
-(d): None of the above
Answer:(c): sp hybridization - When propanoyl chloride reacts with water, what product is formed?
-(a): Propanoic acid and hydrochloric acid
-(b): Propanol and hydrochloric acid
-(c):: Propanamide and hydrochloric acid
-(d):: Propanoate and hydrogen chloride
Answer:(a):: Propanoic acid and hydrochloric acid - Which functional group contains nitrogen as part of its structure?
-(a):: Alcohols
-(b):: Ethers
-(c):: Amines
-(d):: Aldehydes
** Answer: *(c):: Amines
30. Which compound can be formed by the dehydration of an alcohol?**
(a) Alkane
(b) Ether
(c) Alkene
(d) Aldehyde
Answer: (c) Alkene
Class 11 Organic Chemistry-Handwritten Notes
Class 11 S-Block important questions Chemistry-PDF
Class 11 p-Block important questions Chemistry-PDF
Authors
-

About the Author P. Hari Prasad is a highly experienced blogger and content writer with over 10 years of experience in crafting engaging, informative, and SEO-optimized articles. Holding a Master's degree in Chemistry (M.Sc Chemistry ), he brings a unique blend of scientific knowledge and creative storytelling to his work. With expertise in educational topics, career guidance, and technology trends, P. Hari Prasad has helped thousands of readers make informed decisions about their academic and professional journeys. His articles are meticulously researched, ensuring accuracy, relevance, and alignment with Google's E-E-A-T (Experience, Expertise, Authoritativeness, Trustworthiness) guidelines. P. Hari Prasad is passionate about empowering students and parents with actionable insights and practical advice. When he's not writing, you can find him exploring new developments in science and technology or mentoring young writers. For more insightful articles, stay tuned to his blog, where education meets inspiration. WordPress, Make Money Online, News and Technology through this website.
View all posts -

Hello dear Friends.My self Swathi.I am a home maker.Intrested in writing articles.Please do comment in the comment box.
View all posts