class11 chapter 6 notes-Thermodyanamics
Thermodynamics
Some Important Points and Terms of the Chapter
- System and the Surroundings: A system in thermodynamics refers to that
part of universe in which observations are made and remaining universe
constitutes the surroundings. The surroundings include everything other
than the system. System and the surroundings together constitute the
universe. The wall that separates the system from the surroundings is called
boundary
- Types of the System:
- Open System: In an open system, there is exchange of energy and
matter between system and surroundings. - Closed System In a closed system, there is no exchange of matter,
but exchange of energy is possible between system and the
surroundings. - Isolated system In an isolated system, there is no exchange of
energy or matter between the system and the surroundings
- State of a System: The state of a system means the condition of the system
which is described in terms of certain observable properties such as
temp(T), pressure(p), volume (v), etc. of the systems. These properties of a
system are called state variables.
class11 chapter 6 notes-Thermodyanamics
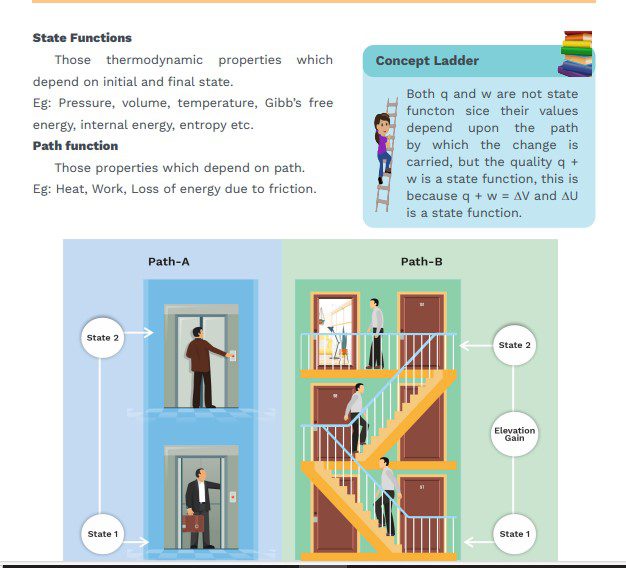
- State Functions: A physical quantity is solid to be state functions of its
value depends only upon the state of the system and does not depend upon
the path by which this state has been attained.
- Internal Energy: a quantity which represents the total energy of the
system. It may be chemical, electrical and mechanical or any other type of
energy you may think of, the sum of all these is the energy of the system. In
thermodynamics, we call it the internal energy, U of the system.
class11 chapter 6 notes-Thermodyanamics
- Isothermal Process: When a process is carried out in such a manner that
the temp remains constant throughout this process, it is called an isothermal
process. - Adiabatic Process: Process is carried out in such a manner that no heat can
flow from the system to the surrounding or vice versa. - Isochoric Process: Process during which the volume of the system is kept
constant. - Isobaric Process: Process during which the pressure of the system is kept
constant. . - The positive sign expresses that Work (Wad) is positive when work is done
on the system. Similarly, if the work is done by the system, wad will be
negative. - The q is positive, when heat is transferred from the surroundings to the
system and q is negative when heat is transferred from system to the
surroundings.
- First law of Thermodynamics: Statement: Energy can neither be created
nor destroyed, however it may be converted from one form to another.
The total energy of the universe remains constant although it may undergo
transformation from one to another.
Mathematical expression U = q + w
- A process or change is said to be reversible, if a change is brought out in
such a way that the process could, at any moment, be reversed by an
infinitesimal change. A reversible process proceeds infinitely slowly by a
series of equilibrium states such that system and the surroundings are
always in near equilibrium with each other. Processes other than reversible
processes are known as irreversible processes.

Zeroth Law of Thermodynamics
If two bodies A and B are separately in thermal equilibrium with thirtli body C, then bodies A
and B will be in thermal equilibrium with each other.
class11 chapter 6 notes-Thermodyanamics
Triple Point of Water
The values of pressure and temperature at which water coexists in equilibrium in all three states
of matter, i.e., ice, water and vapour
called triple point of water.
Triple point of water is 273 K temperature and 0.46 cm of mere pressure.
Specific Heat
The amount of heat required to raise the temperature of unit mass the substance through 1°C is
called its specific heat.
It is denoted by c or s.
The specific heat of water is 4200 J kg’°C-‘ or 1 cal g‘ C, which high compared with most
other substances.
Gases have two types of specific heat
- The specific heat capacity at constant volume (Cv).
- The specific heat capacity at constant pressure (C,).
Specific heat at constant pressure (Cp) is greater than specific heat constant volume (Cv), i.e.,
Cp > Cv •
For molar specific heats Cp – Cv = R
where R = gas constant and this relation is called Mayer’s formula.
The ratio of two principal sepecific heats of a gas is represented by gamma.
The value of gamma depends on atomicity of the gas.
Amount of heat energy required to change the temperature of any substance is given by
Q = mcAt
Thermal (Heat) Capacity
Heat capacity of any body is equal to the amount of heat energy required to increase its
temperature through I°C.
Heat capacity = me
where c = specific heat of the substance of the body and m = mass of the body.
Its SI unit is joule/kelvin (J/K).
Water Equivalent
It is the quantity of water whose thermal capacity is same as the heat capacity of the body. It is
denoted by W.
W = ms = heat capacity of the body.
Latent Heat
The heat energy absorbed or released at constant temperature per unit mass for change of state
is called latent heat.
Heat energy absorbed or released during change of state is given by
Q = mL
where m = mass of the substance and L = latent heat.
It is more painful to get burnt by steam rather than by boiling was 100°C gets converted to
water at 100°C, then it gives out 536 heat. So, it is clear that steam at 100°C has more heat than
wat 100°C (i.e., boiling of water).
After snow falls, the temperature of the atmosphere becomes very This is because the snow
absorbs the heat from the atmosphere to down. So, in the mountains, when snow falls, one does
not feel too but when ice melts, he feels too cold.
There is more shivering effect of ice cream on teeth as compare that of water (obtained from
ice).
This is because when ice cream down, it absorbs large amount of heat from teeth.
Melting
Conversion of solid into liquid state at constant temperature is melting.
Evaporation
Conversion of liquid into vapour at all temperatures (even below boiling point) is called
evaporation.
🙂🙂🙂🙂🙂🙂🙂🙂🙂🙂🙂🙂🙂🙂🙂🙂🙂🙂🙂🙂🙂🙂🙂🙂🙂🙂🙂
Author
-

Hello dear Friends.My self Swathi.I am a home maker.Intrested in writing articles.Please do comment in the comment box.
View all posts
