State and explain Hess Law of constant Heat summation ?
Answer : Hess Law: ” The total heat change in a reaction is the same,whether the chemical reaction is the same,whether the chemical reaction takes place in a single step or in serveral steps “.
It is based on the Ist law of thermodynamics.
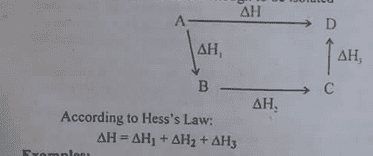
Explanation : Consider a reaction A—->D.
Suppose that D is formed from A in two different paths. Path ① and Path ②
Path ①: A → D, ΔH
Total heat change in path ①=ΔH
Path ②: A → B : ΔH1
B→C :ΔH2
C→D: ΔH3
Total heat change in path ② is
ΔH1+ΔH2+ΔH3
Now ,from the Hess law,we have
ΔH = ΔH1 +Δ2 +ΔH3
3) Example:
C(graphite) +O2( g)→CO2( g) ; ΔH=−393.5KJ mol-1
C(graphite) +1/2O2( g)→CO(g); ΔH1=−110.5KJ
CO(g)+1/2O2( g)→CO2( g) ; ΔH2=−283.02KJmol-1
Total heat change in path② =ΔH1+ΔH2
=(−110.5)+(−283.02)=−393.52KJ.
Thus ΔH=ΔH1+ΔH2. Hence the Hess law is proved
PROBLEMS IN Hess Law
1. The enthalpies of formation CO (g), CO2 (g), N2O (g) and N2O4 (g) are − 110, − 393, 81 and 9.7 KJ/mol, respectively. Find the value of ΔH for the reaction.
N2O4 (g) + 3 CO → N2O (g) + 3 CO2 (g).
Answer: ΔHo = ΔHo(products) − ΔHo(reactants)
ΔH = 3 ΔCO2 + ΔN2O − 3 ΔCO − Δ N2O4
ΔH = 3 × (−393) + (81) − 3 × (−110) − (9.7)
ΔH = − (777.7) KJ/mol
2.. Calculate the enthalpy change for the following reaction:
CH4 (g) + 2 O2 (g) ⟶ CO2 (g) + 2 H2O (l).
Given that enthalpies of formation of CH4, CO2 and H2O are 74.8 kJmol−1,− 393.5 kJ mol−1, and − 286 kJmol−1, respectively.
Answer: ΔHo = ΔHo(products) − ΔHo(reactants)
ΔHo = [ΔHo(CO2) + 2 X ΔHo(H2O)] − [ΔHo(CH4) + 2 X ΔHo(O2)]
ΔHo = [− 393.5 + 2 X (−286.2)] − [−74.8 + 2 X 0]
ΔHo = − 393.5 – 572.4 + 74.8
ΔHo = − 891.1 kJ/mol
Hess’s Law Quiz
Author
-

About the Author P. Hari Prasad is a highly experienced blogger and content writer with over 10 years of experience in crafting engaging, informative, and SEO-optimized articles. Holding a Master's degree in Chemistry (M.Sc Chemistry ), he brings a unique blend of scientific knowledge and creative storytelling to his work. With expertise in educational topics, career guidance, and technology trends, P. Hari Prasad has helped thousands of readers make informed decisions about their academic and professional journeys. His articles are meticulously researched, ensuring accuracy, relevance, and alignment with Google's E-E-A-T (Experience, Expertise, Authoritativeness, Trustworthiness) guidelines. P. Hari Prasad is passionate about empowering students and parents with actionable insights and practical advice. When he's not writing, you can find him exploring new developments in science and technology or mentoring young writers. For more insightful articles, stay tuned to his blog, where education meets inspiration. WordPress, Make Money Online, News and Technology through this website.
View all posts

