P BLOCK ELEMENTS IPE 2025 IMPORTANT QUESTIONS-P BLOCK ELEMENTS
P- BLOCK ELEMENTS-2 Marks
1.Allotropy : If an element exists in different physical forms but shows same chemical properties ,this property is called ALLOTROPY
eg.Diamond,Graphite,Fullerene.
——————————————————————————-
2.Hybridisation of Carbon in
1.CO3-2 = SP2 4.Diamond = SP3
2.Graphite = SP2
3.Fullerene = SP2
——————————————————————————-
3.Man made silicates -🡪1.Glass and 2. Cement
——————————————————————————-
4.CO is poisonous because it combines with Hb and forms
“CARBOXY HAEMOGLOBIN “
Hb + Co 🡪 Hb-Co
It is 300 times more stable than oxy haemoglobin.
———————————————————————-
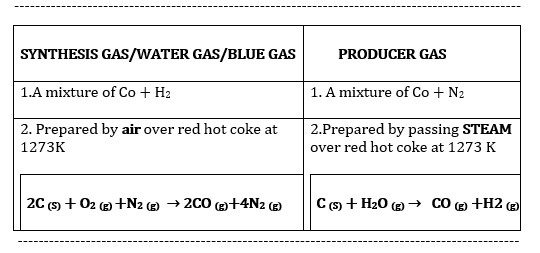
5.What is Water gas and Producer gas ?
P BLOCK ELEMENTS IPE 2025 IMPORTANT QUESTIONS
——————————————————————————-
|
SYNTHESIS GAS/WATER GAS/BLUE GAS |
PRODUCER GAS |
||
|
1.A mixture of Co + H2 |
1. A mixture of Co + N2 |
||
|
2. Prepared by air over red hot coke at 1273K
|
2.Prepared by passing STEAM Over red hot coke at 1273 K |
——————————————————————————-
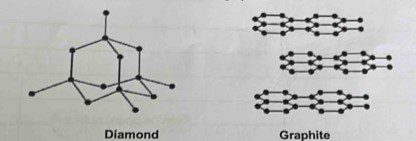
6.Diamond is hard because
1.SP3 Hybridisation
2.It has 3D structure.
3.Each carbon is linked to 4 other carbon atoms in “TETRAHEDRAL “manner
4.Energy required to break the bond is very high (348 KJ/mol )
———————————————————————–
7.Graphite act’s as Good Conductor and Lubricant because::
1. Soft,Slippery in nature.
2. SP2 hybridisation is present
3.Weak vanderwaals forces are present.
4.Layers slide one over the other
5.Free electrons are present in P-Orbital
P BLOCK ELEMENTS IPE 2025 IMPORTANT QUESTIONS
——————————————————————————
8. DRY ICE ::
Solid CO2 is called as DRY ICE .Used as
①Fire Extinguisher ② .Manufacture of urea ③Refrigerant for ice-creams.
——————————————————————————-
9.INERT PAIR EFFECT : The ns electrons doesn’t show intrest in bond formation is called “INERT PAIR EFFECT “
OR
The reluctance of ns electrons to take part in bond formation is called inert pair effect
Ex: Lead (Pb) shows +2 oxidation state instead of +4 oxidation state.
Thallium (Tl) shows +1 oxidation state instead of +3 oxidation state.
——————————————————————————-
10.Silicones : Organo Silicon Polymers containing Si-O-Si linkage.
2. Contains R2SiO repeating units
3.Structure similar to Ketones.
Uses :
——————————————————————————-
11. ZSM-5
1. Zeolite Socony Mobil-5
2.It is a type of Zeolite.
3.Used for conversion of Alcohols into Gasoline directly.
—————————————————————————–
12.BF3 act’s as a Lewis acid because :
①It is an electron deficient compound
②The central atom B has 6 electrons,it
requires 2 electrons to get Octet Configuration .
③According to Lewis ,Acid means,
electron pair acceptor hence BF3 act’s as Lewis Acid
——————————————————————————-
13. INORGANIC BENZENE :–
①Borazine is called as “INORGANIC BENZENE”
②Its formula is B3N3H6
③Its structure is similar to BENZENE
P BLOCK ELEMENTS IPE 2025 IMPORTANT QUESTIONS
——————————————————————————-
14.Diagrams of Diamond & Graphite
P BLOCK ELEMENTS IPE 2025 IMPORTANT QUESTIONS
Author
-

About the Author P. Hari Prasad is a highly experienced blogger and content writer with over 10 years of experience in crafting engaging, informative, and SEO-optimized articles. Holding a Master's degree in Chemistry (M.Sc Chemistry ), he brings a unique blend of scientific knowledge and creative storytelling to his work. With expertise in educational topics, career guidance, and technology trends, P. Hari Prasad has helped thousands of readers make informed decisions about their academic and professional journeys. His articles are meticulously researched, ensuring accuracy, relevance, and alignment with Google's E-E-A-T (Experience, Expertise, Authoritativeness, Trustworthiness) guidelines. P. Hari Prasad is passionate about empowering students and parents with actionable insights and practical advice. When he's not writing, you can find him exploring new developments in science and technology or mentoring young writers. For more insightful articles, stay tuned to his blog, where education meets inspiration. WordPress, Make Money Online, News and Technology through this website.
View all posts