State and explain Hess Law of constant Heat summation ?
Answer : Hess Law: ” The total heat change in a reaction is the same,whether the chemical reaction is the same,whether the chemical reaction takes place in a single step or in serveral steps “.
It is based on the Ist law of thermodynamics.
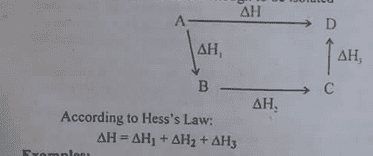
Explanation : Consider a reaction A—->D.
Suppose that D is formed from A in two different paths. Path ① and Path ②
Path ①: A → D, ΔH
Total heat change in path ①=ΔH
Path ②: A → B : ΔH1
B→C :ΔH2
C→D: ΔH3
Total heat change in path ② is
ΔH1+ΔH2+ΔH3
Now ,from the Hess law,we have
ΔH = ΔH1 +Δ2 +ΔH3
3) Example:
C(graphite) +O2( g)→CO2( g) ; ΔH=−393.5KJ mol-1
C(graphite) +1/2O2( g)→CO(g); ΔH1=−110.5KJ
CO(g)+1/2O2( g)→CO2( g) ; ΔH2=−283.02KJmol-1
Total heat change in path② =ΔH1+ΔH2
=(−110.5)+(−283.02)=−393.52KJ.
Thus ΔH=ΔH1+ΔH2. Hence the Hess law is proved
PROBLEMS IN Hess Law
1. The enthalpies of formation CO (g), CO2 (g), N2O (g) and N2O4 (g) are − 110, − 393, 81 and 9.7 KJ/mol, respectively. Find the value of ΔH for the reaction.
N2O4 (g) + 3 CO → N2O (g) + 3 CO2 (g).
Answer: ΔHo = ΔHo(products) − ΔHo(reactants)
ΔH = 3 ΔCO2 + ΔN2O − 3 ΔCO − Δ N2O4
ΔH = 3 × (−393) + (81) − 3 × (−110) − (9.7)
ΔH = − (777.7) KJ/mol
2.. Calculate the enthalpy change for the following reaction:
CH4 (g) + 2 O2 (g) ⟶ CO2 (g) + 2 H2O (l).
Given that enthalpies of formation of CH4, CO2 and H2O are 74.8 kJmol−1,− 393.5 kJ mol−1, and − 286 kJmol−1, respectively.
Answer: ΔHo = ΔHo(products) − ΔHo(reactants)
ΔHo = [ΔHo(CO2) + 2 X ΔHo(H2O)] − [ΔHo(CH4) + 2 X ΔHo(O2)]
ΔHo = [− 393.5 + 2 X (−286.2)] − [−74.8 + 2 X 0]
ΔHo = − 393.5 – 572.4 + 74.8
ΔHo = − 891.1 kJ/mol